Pharma Product, Literature & Compliance
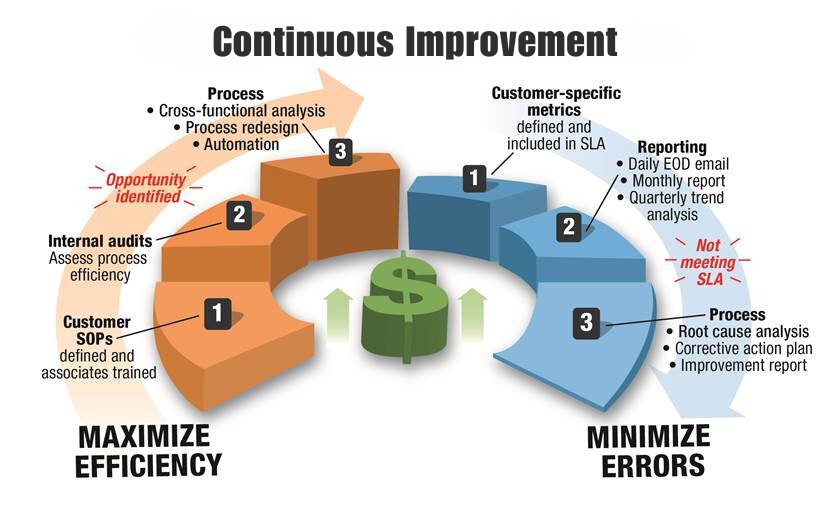
Staci Americas' quality control team leverages a full arsenal of continuous improvement tools and techniques.

eDisclosure
We keep track of all the ‘fine print’ in one dynamic database and automatically insert the right copy each time a user places an order.
eWaiver
We generate a pop-up reminder of usage or distribution restrictions for affected documents, then track and save the required data.
Dynamic Database
Create dynamic links to your product and supporting documents, including those not produced, stored or fulfilled by Staci Americas.
Document Lifecycle
We ensure that every version of documents in your inventory is searchable and readily available until it is securely destroyed at the date you specify.
Staci Americas 360TM system automates compliance tasks to protect your brand. Read the case study on how our online fulfillment system addressed productivity and compliance issues for a major pharmaceutical company.
Our system allows fast, easy, online ordering and provides real-time updates on orders and inventory 24/7.
Customization capabilities that allow you to make communications more relevant to individual recipients.
Six Sigma
Learn methodologies are applied in all Staci logistics processes.
Analysis
Explore the impact of layout or process changes on fulfillment cost and service.
Benchmarking
Using our “DC Expert” tool.
Reporting
Real-time reports with KPIs can be scheduled or downloaded, on demand, using our web portal.
KPI Management
A wide range of metrics, such as inventory accuracy, order accuracy, on-time delivery, average lines per order, and average orders per hour can be monitored and reported, based on your requirements.
GM Control
Each Staci Americas facility maintains a separate P&L, and facility General Managers are incented to drive quality improvements and control costs
"What if" Analysis
Explore the impact of layout or process changes on fulfillment cost and service.
Benchmarking
Reporting
KPI Management
GM Control
Six sigma
Ready to get started?
We are here to answer your questions

